Making AI-Powered Healthcare Mainstream: What the Health Tech Investment Act (S.1399) Means for AI-Device Developers
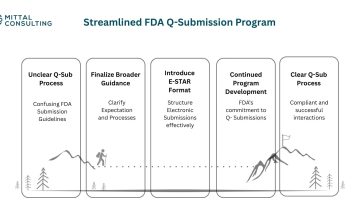
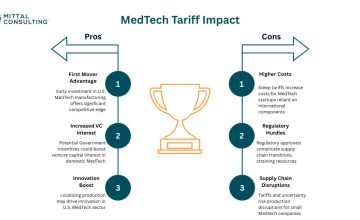
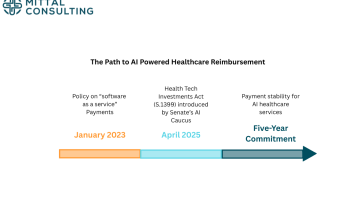
By- Anya Bekhtel (Mittal Consulting) Artificial intelligence and machine learning are driving innovation throughout healthcare, creating opportunities for faster and more accessible treatment solutions. While the FDA has authorized hundreds of AI and ML enabled devices over the last five years, the pathway to reimbursement for these systems has remained unclear. The Senate Artificial Intelligence […]