FDA Launches Regulatory Accelerator to Empower Digital Health Innovators
By: Anya Bekhtel (Mittal Consulting)
FDA Launches Regulatory Accelerator to Empower Digital Health Innovators
On July 24, 2025, the U.S. Food and Drug Administration (FDA) unveiled Regulatory Accelerator, an initiative intended to empower innovators on the journey of bringing digital health products to the US market.
Regulatory Accelerator: A Centralized Resource for Success
Regulatory Accelerator is a user-friendly hub of FDA resources for digital health device innovators. At time of launch, Regulatory Accelerator includes:
1. Resource Index for Digital Health Device Innovators
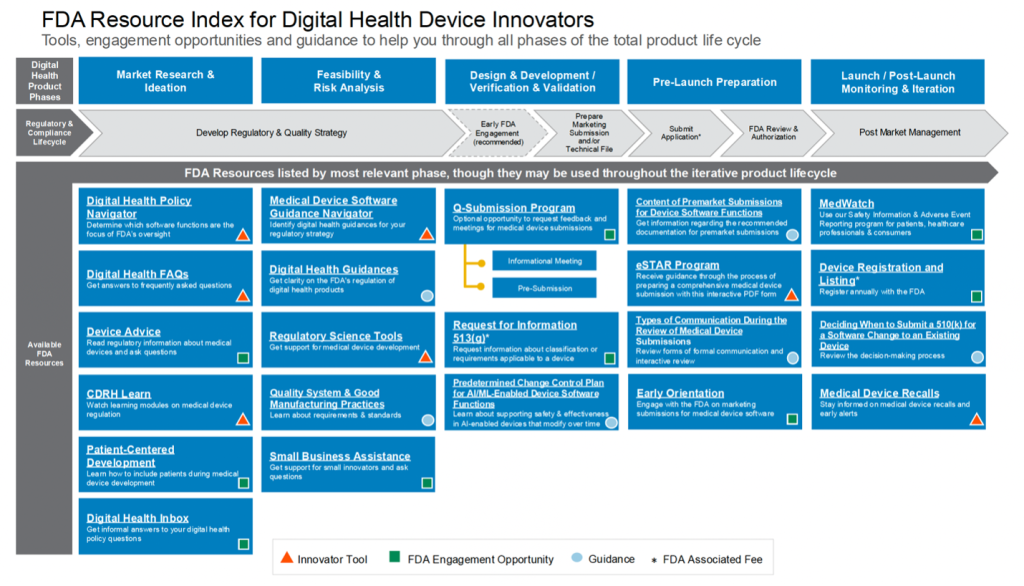
The Resource Index for Innovators is a visual, stepwise guide to core FDA tools, engagement opportunities, and relevant guidance documents mapped across the device development and compliance lifecycle. The Index notes six (6) free opportunities for pre-launch FDA engagement as well as the three (3) steps associated with an FDA fee: the 513(g) Request for Medical Device Designation, Application Submission, and Device Listing and Registration.
FDA Resource Index for Digital Health Device Innovators
2. Early Orientation Meetings
Early Orientation Meetings are optional meetings that allow sponsors to engage with the FDA review team early in the marketing submission process. FDA’s new “Best Practices for Early Orientation Meetings for Marketing Submissions for Medical Device Software” can help innovators prepare for device introductions and product demonstrations to the FDA that can best inform FDA reviewers of how a device achieves its intended use. This early dialogue can strengthen sponsor-regulator collaboration and improve FDA’s product understanding to increase efficiency within the review process.
3. Medical Device Software Guidance Navigator
The Medical Device Software Navigator tool streamlines identification of pertinent FDA guidance documents for medical device software products. This tool was designed to aligned closely with the electronic Submission Template and Resource (eSTAR) used for many FDA marketing submissions, including guidance on:
- Submission Type
- Pre-Submission Correspondence
- Consensus Standards
- Device Description
- Proposed Indications for Use
- Classification
- Predicates and Substantial Equivalence
- Software
- Artificial Intelligence
- Cybersecurity
- Interoperability
- Performance Testing
- Human Factors
Supporting Innovation and Patient Access
The debut of Regulatory Accelerator marks an advancement in FDA’s support of digital health innovation. By integrating tools under the Regulatory Accelerator umbrella into their regulatory strategy, innovators can more confidently navigate FDA processes to deliver lifesaving treatments and technologies to US patients.