Siloam Vision Earns Breakthrough Device Designation for OCT Technology Aimed at Preventing Infant Blindness
May 14, 2025
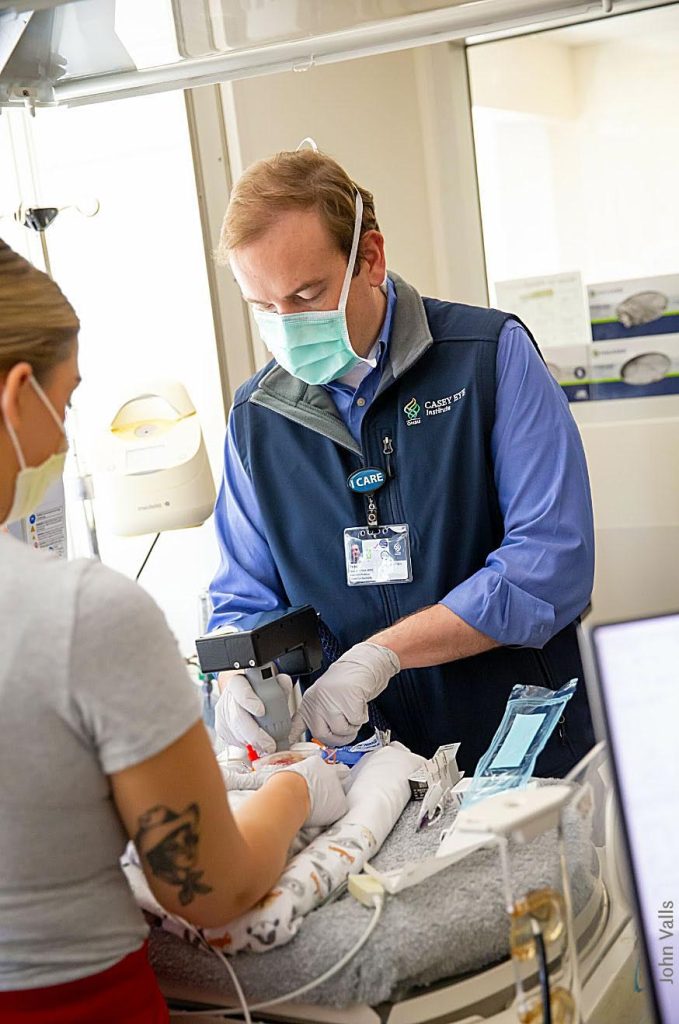
Since 2021, Siloam Vision has been pioneering clinical solutions to prevent blindness and improve global vision health. Retinopathy of Prematurity (ROP) is a leading global cause of permanent infant blindness, particularly in regions with limited access to adequate eye care. Driven by the belief that no child should lose their sight to a preventable disease, the founders of Siloam Vision set out to transform the eye care landscape by developing innovative, low-cost equipment and software to combat the irreversible effects of ROP.
At the end of April 2025, Siloam Vision achieved a major regulatory milestone in their mission to prevent infant blindness: the company received the coveted Breakthrough Device Designation from the United States Food and Drug Administration (US FDA) for their iCam OCT camera. This designation marks the second Breakthrough Device Designation awarded to Siloam Vision; the first was granted for their AI-based plus disease detection device, underscoring the company’s continued innovation and leadership in ROP diagnostics.

Siloam Vision partnered with Mittal Consulting to pursue the designation. The two teams worked synergistically to develop a strategic and compelling Breakthrough submission that demonstrated the true clinical value and innovative nature of the iCam in ROP care. Following a three-month collaborative drafting process and a responsive, team-driven approach to FDA requests, Siloam received the Breakthrough Designation just 60 calendar days after submission in the first attempt.
The FDA’s Breakthrough Devices Program is a voluntary program for certain medical devices and device-led combination products that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. The goal of the program is to provide patients and health care providers with timely access to these medical devices by speeding up their development, assessment and review, while preserving the statutory standards for premarket approval, 510(k) clearance and De Novo marketing authorization, consistent with the Agency’s mission to protect and promote public health.
Mittal Consulting firmly believes in a hands-on, collaborative approach to all FDA submissions. We synergistically combine the breadth of technical and clinical expertise from our clients with our internal team’s rigorous regulatory knowledge to craft and submit high-quality, tailored applications aimed at the highest level of success.
With Breakthrough Designation for the iCam OCT now secured, Siloam Vision and Mittal Consulting are collaboratively working to prepare a comprehensive marketing application for FDA clearance, anticipated in 2026. Mittal Consulting is proud to represent Siloam Vision and support their efforts to overcome regulatory hurdles in their mission to end childhood blindness.

